SUPPLEMENTAL EFFECT OF CONDENSED TANNINS FROM SENGON LEAVES (ALBIZIA FALCATARIA) ON IN VITRO GAS AND METHANE PRODUCTION
Afzalani, A*., R. A. Muthalib, R. Raguati, E. Syahputri, L. Suhaza and E. Musnandar
Faculty of Animal Science, University of Jambi, Jambi Indonesia 36361
* Corresponding Author: afzalani@unja.ac.id
ABSTRACT
The present study was conducted to evaluate the effect supplementation of sengon leaves (SL) with a condensed tannin (CT) content on the in vitro profile of cumulative gas production (GP), methane (CH4) production, and rumen fermentation parameters. The SL containing 8.84% CT (88,4 mg per g SL) was supplemented at different levels of T0, T1, T2, T3 (0, 2, 4, and 6% CT per dry matter incubation of basal ration, respectively) during 48 h fermentation in 120 ml of serum bottles. The basal ration (BR) consisted of Brachiaria mutica grass and concentrate mix (60:40 ratio). Rumen fluid was collected from ruminally fistulated Bali cattle (Bos sondaicus). The profile of cumulative gas and methane production was fitted using y= a+ b (1 – e-ct). The profile of cumulative gas and methane production was lower at T3 and T2 than at T1 and T0. Methane gas production stabilized after 24 h of incubation for T3 and T2. Meanwhile, the profile of methane GP was stabilized after 36 h for T1 (2% CT) and after 48 h for T0 (0% CT) (control). The fermentation parameters measured in this study showed that increased supplementation with CT-SL significantly (P<0.05) decreased the in vitro dry matter digestibility (IVDMD), in vitro organic matter digestibility (IVOMD), ammonia (N-NH3), total volatile fatty acid (TVFA), total gas (TG, ml), total methane (ml) and methane percentage (CH4 per TG). The study concluded that supplementation of CT-SL at T2 (4% CT equal to 0.09 g SL/0.2 g BR, or equal to supplementation of 45% SL per BR (DM basis) was more effective in controlling methane production and was still favorable in IVDMD, IVOMD, N-NH3, and TVFA to maintain ruminal microbial activities and ruminant needs. The results suggest that evaluation under in vivo conditions is needed.
Key words: supplementation, sengon leaves, condensed tannins, methane, in vitro
https://doi.org/10.36899/JAPS.2022.6.0559
Published first online June 11, 2022
INTRODUCTION
Methane emitted from livestock primarily contributes to the total greenhouse gas (GHG)in the atmosphere. The methane emitted from ruminants can cause dietary gross energy loss up to2-12%and represent globally 2,779 and 2,344 Mt CO2-e/year in 2010 and 2020, respectively (Hristov et al., 2013; Subepang et al., 2019). In Indonesia, the agricultural sector was the third-highest emitting sector, with a total emission of 132 Mt CO2-ein 2005, which is 13.6% of the national GHG emissions. It will grow up to 25 percent to 164 Mt CO2-ein 2030 (NCCC, 2010). Methane emissions from the agriculture sector accounted for seventy-one percent of the emissions from rice fields, whereas livestock contributed to 16.5% of methane emissions from the agricultural sector. Emissions from the livestock sector are expected to increase from 25 Mt CO2-e to 39 Mt CO2-e. This condition is estimated to cause an increase of more than 50 percent in additional emissions (NCCC, 2010). The Indonesian government has planned to abate 105 Mt CO2-e by 2030 from agriculture, including the livestock sector, by improving water management in rice cultivation, the restoration of degraded land and feed supplementation or feeding strategies for livestock (NCCC, 2010).
An approach to reduce methane production from ruminants has been conducted and shown to be effective using natural compounds in plants (Cottle et al., 2011; Jayanegara et al., 2014). In the tropics, as in Indonesia, there is a diversity of plants that, due to their good nutritive value, hold potential for ruminant feeding (Bhatta et al., 2013). Plants rich in condensed tannins can decrease bacterial and protozoal populations in the rumen as well as CH4 emissions (Puchala et al., 2012). Some research on the mitigation of CH4 production and improvement of ruminant performance has been done by supplementation of tree foliage with tannin content (Kennedy and Charmley 2012; Anas et al., 2015; Gemeda and Hassen, 2015; Min et al., 2015).
Feeding condensed tannin-containing forages or feedstuffs to ruminants may be an effective natural practice for mitigating CH4 emissions by ruminant livestock and increasing metabolizable energy intake (Naumann et al., 2017). The action of tannins in methane production directly prevents growth and methanogen activity (Tavendale et al., 2005) and indirectly creates complexes with polysaccharides and proteins by limiting access to methanogens (Naumann et al., 2017). Nonetheless, the voluntary intake will decrease if the intake of condensed tannins is above 7% of the dry matter (DM). Moderate intake of condensed tannins (3-6% of DM ratio) may also lead to positive responses (Vázquez et al., 2016).
Sengon (Albizia falcataria) is a tree foliage of legumes planted to be used by the wood, paper, and paper tissue industries. Laboratory analysis has found that sengon leaves contain a high polyphenol compound as well as a condensed tannin content of 8.84% dry matter (DM).
Based on this finding, the purpose of the present study was to determine the supplementation effect of Sengon leaves (SL) (Albizia falcataria) as a condensed tannin source on in vitro gas and methane production.
MATERIALS AND METHODS
Sample Collection, Extraction and condensed tannin Analysis: Sengon leaves (SL) samples were obtained from the teaching and research farm of the University of Jambi, Faculty of Animal Science, Indonesia.The sample was air-dried indoors, oven dried for 24 hours at 60°C and ground to pass through a 1 mm sieve before condensed tannin (CT) analysis and in vitroincubation.
Extraction was carried out using maceration techniques with 95% methanol as a solvent. A total of 100 g of sample and 300 ml of methanol (1:3 w/v) were put into a glass beaker and soaked for 12 hours. The extract obtained was filtered with four-layer cloths. The filtrate obtained was then concentrated using a rotary evaporator and dried in an oven at 40°C to a constant weight. (Mayangsari et al., 2013).
To determine the condensed tannin (CT) fraction, the extract was treated with butanol-HCl in the presence of ferric ammonium sulfate, and CT was expressed as leucocyanidin equivalents as follows:
CT= (A550 nm×782.6)/sample weight (DM), where A550 nm is the absorbance valueat 550 nm. Assuming that the effective E1 cm, 550 nm of leucocyanidin was 460 (Porter et al. 1986).
In vitro Rumen Fermentation and GP Measurement: The basal rations (BR) used in this study were prepared with a protein content of ±13% and a TDN of ± 65% with Brachiaria mutica (BM) grass (60%) and concentrate mix (40%). The concentrates were formulated using 58% rice bran, 25% ground corn, 6% soybean meal, 9% coconut cake, 1% mineral mix, and 1% salt. The ration mixtures (DM basis) were dried at 60°C for 12 hours and ground with a 1 mm sieve. The nutrient composition of feeds and basal ration (BR) arepresented in Table 1.
Tabel.1. Nutrient Composition of Brachiaria mutica, Sengon Leaves, Concentrate and Basal Ration
|
Nutrients
|
Brachiaria mutica
|
Sengon Leaves
|
Concentrate
|
Basal Ration
|
|
DM, %
|
92.25
|
92.02
|
91.48
|
91.94
|
|
CP, %
|
12.94
|
14.07
|
12.47
|
12.75
|
|
CF, %
|
27.67
|
14.64
|
8.81
|
20.13
|
|
EE, %
|
1.37
|
3.61
|
3.64
|
2.28
|
|
NFE, %
|
50.13
|
63.21
|
68.74
|
57.57
|
|
NDF, %
|
71.98
|
51.22
|
50.85
|
63.53
|
|
ADF, %
|
34.65
|
25.86
|
14.78
|
26,70
|
|
Ash, %
|
7.89
|
4.47
|
6.34
|
7.27
|
|
TDN, %
|
56.76
|
72.41
|
78.32
|
65.38
|
|
CT, %
|
-
|
8.84
|
-
|
-
|
DM; Dry matter, CP; Crude protein, CF; Crude fiber, EE; Ether extract, NFE; Nitrogen free extract, NDF; Neutral detergent fiber, ADF; Acid detergent fiber, CT; Condensed tannins
The Theodorou and Brooks (1990) method was applied for the in vitro incubation. Approximately 0.2 g sample ration was scaled into a 120 ml bottle of serum to be kept in a 39°C incubator. Rumen fluid was obtained from fistulated Bali cattle in the morning before feeding time, which was then brought to the laboratory, strained using four layers of cheesecloth, and then mixed with McDougall buffer solution (1:4 v/v) according to the procedure in Tilley and Terry (1963). A total of 2000 ml of a mixture of rumen fluid and McDougall buffer solution was put into a 2500 ml dark bottle, saturated with CO2, and then installed with an automatic dispenser pipette. Condensed tannins (CTs) were added and incubated in a serum bottle according to the following treatments: T0: basal ration (BR), T1: T0 + 2% CT (equal to 0.05 g SL/0.2 g BR, dry feed basis), T2: T0 + 4% (equal to 0.09 g SL/0.2 g BR, dry feed basis), and T3: T0 + 6% CT (equal to 0.14 g SL/0.2 g BR, dry feed basis). An amount of 40 ml rumenbuffer solution was placed in each serum bottle. The bottles were sealed with rubber stoppers and crimp seal caps. A syringe was used to inject a small amount of gas into the serum bottle through the rubber stopper to initiate the point of incubation. A total of 51 serum bottles for 5 serum bottles of each sample treatment and 3 serum bottles as blanks (i.e., Rumen fluid only), were incubated in an incubator at 39°C for 48 h.
The volume of GPwas recorded manuallyat 2, 4, 8, 16, 24,and48h after incubation by an inserted needle with a 10 ml gas syringe through the rubber stopper. GP was read by looking at changes in the scale of the gas syringe (Fortune®). Total gas value were corrected the blank. This study measured methane GP based on the Fievez et al. (2005) method. The total gas produced was flown into 4 M NaOH. carbon dioxide, which was the main gas produced during in vitro rumen fermentation, was then bound to NaOH. Another syringe connected to the system was used to read the methane volume. Methane gas production was corrected with a blank, and the methane concentration was determined as:

The methane reduction potential (MRP) was calculated by taking the net methane value for the control (basal ration without CT) as 100%:
MRP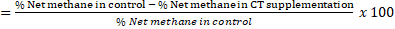
A total of 51 serum bottles for 2 serum bottles of each sample treatment and 3 serum bottles as blanks (i.e., Rumen fluid only) were incubated in an incubator at 39°C for 48 h. At the end of 48 h after fermentation, the pH was measured to determine the final pH using pH matter and then centrifuged at 6000×g for 15 min to obtain residues for the determination of in vitrodry matter digestibility (IVDMD) and in vitro organic matter digestibility (IVOMD). Meanwhile, the supernatant was used to determine total VFA and ammonia-nitrogen (N-NH3). The IVDMD and IVOMD were measured using the procedures of AOAC (2007). The TVFA was determined using steam distillation and N-NH3 with microdiffusion Conway according to the General Laboratory procedure (1966).The profile of cumulative gas and methane production was fitted to the model of Ørskov and McDonald (1979) as follows:
y = a+ b (1 – e-ct)
where a is the gas and methane production from immediately soluble fraction, b the gas and methane production from the insoluble fraction, c the gas and methane production rate constant for insoluble fraction (b), t the incubation time, and y the gas and methane produced at time ‘t’.
Statistical Analysis: The experiment was conducted in a completely randomized design (CRD) that consisted of four treatments and five replicates. Each replicate was represented by two incubation bottles. Data obtained from the experiment were analyzed using analysis of variance (ANOVA). Comparisons among treatments were performed by applying Duncan's multiple range test with a significant difference at P<0.05. The data were analyzed using SPSS statistical software version 17.0.
RESULTS AND DISCUSSION
Profile of Gas Production (GP): The effect of supplementation of SL with CT content on the profile of GP is presented in Figure 1. It can shown (Figure 1) that the length of incubation time tends to increase the cumulativeGP, where is because of the increased total substrate fermented by rumen microbes. However, supplementation of SL with a CT content had affected in reducing total GP. The average GP obtained in this experiment ranged from1.06-1.55 ml per hour. The rate of GP was decreasing in line with the increased incubation time and level of CTsupplementation. GP at 48 h varied between 50.17 - 73.47 ml, with the highest being for T0 (without CT) and lowest for T2 (4% CT) andT3 (6% CT).

Figure1. The Effect supplementation of sengon leaves with condensed tannin content on cumulative gas production (ml)
GP after fermentation greatly indicates the number of available carbohydrates as the energy source for rumen microorganisms (Ushlu et al., 2018). The extent of GP depends on the amount of fermentable carbohydrates, but the presence of secondary metabolite substances such as tannins may also influence it (Jayanegara et al., 2014; Kondo et al., 2014; Bueno et al., 2020). Tannins are also found to decrease GP in the in vitro system by forming a tannin-macromolecule complex, which inhibits the activities of the microbial fibrolytic enzyme. CT is able to reduce the fermentation and digestibility of organic matter found in the rumen by altering the proportion of VFAs, particularly the acetate:propionate ratio. Under in vitroconditions, it increases the molar proportion of propionate without unaffecting the molar proportion of acetate (Jayanegara et al., 2012; Hassanat and Benchaar, 2013; Ningrat et al., 2017; Bueno et al., 2020).
Profile of Gas Methane: The effect of supplementation of SL with a CT content on the profile of methane GP is presented in Figure 2.
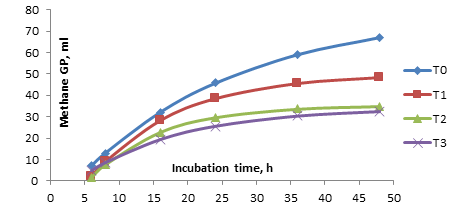
Figure 2. The effect supplementation of sengon leaves with condensed tannin content on methane gas production (ml)
In general, from figure 2 above, all treatments increased methane production during early incubation at 6-16 h and then began a decline after 16 h, as shown by the slope of the curve and stabilized after 24 h, especially for the T2 (4% CT) and T3 (6% CT) treatments. Meanwhile, the methane GP profile was stabilized after 36 h for T1 (2% CT) and after 48 h for T0 (0% CT) (control). This shows that methanogens are more active at the early incubation stage but less active as the fermentation substrate is reduced. However, it appears that supplementation of feed containing CT tends to reduce methane GP. The specific effect of CT on the reduction in methane emissions is unknown (Piñeiro-Vázquez, et al., 2015), but other studies have pointed out that they form complexes with dietary proteins and cabohydrates in the rumen, thus decrease the DMD and OMD and indirectly affect the release of H2 (Jayanegara et al., 2011; Bueno et al., 2020).Rumen Fermentation Parameters: The fermentation parameters (pH, IVDMD, IVOMD, N-NH3, total VFA, total gas, total methane and methane reduction potency) are presented in Table 2.Tabel.2. Effect of supplementation sengon leaves with condensed tannin content on rumen fermentation parameters
| Parameters |
Treatments |
SEM |
p value |
| T0 |
T1 |
T2 |
T3 |
| pH |
6.92 |
6.92 |
6.90 |
6.86 |
0.033 |
<0.487 |
| IVDMD (%) |
62.738a |
56.08b |
50.28c |
39.68d |
1.736 |
<0.001 |
| IVOMD (%) |
62.86a |
47.41b |
44.450b |
36.382c |
1.285 |
<0.001 |
| N-NH3 (mM) |
6.45a |
3.93b |
3.59c |
3.26c |
0.173 |
<0.001 |
| TVFA (mM) |
178.00a |
161.00b |
150.00c |
123.00d |
3.122 |
<0.001 |
| TG (ml) |
74.45a |
64.30b |
52.98c |
51.10c |
0.933 |
<0.001 |
| CH4 (ml) |
66.47a |
47.982b |
33.53c |
31.03c |
0.947 |
<0.001 |
| CH4 (% TG) |
89.29a |
74.63b |
63.29c |
60.63d |
0.821 |
<0.001 |
| MRP (%) |
- |
27.82 |
49.56 |
53.32 |
- |
- |
a, b, c,d Different superscript letters in each row indicate significant differences at p<0.05.T0 to T3 were treatments supplemented with 0, 2, 4, and 6% condensed tannin-sengon leaves from dry matter of feeds. IVDMD = in vitro dry matter digestibility, IVOMD= in vitro organic matter digestibility, N-NH3= N-ammonia, TVFA= total volatile fatty acids, TG= total gas, CH4= gas methane, MRP= methane reduction potency.
Ruminal pH is a fermentation parameter that quantifies the state of acidity and alkalinity of the rumen. The results in Table 2 indicate that supplementation of SL with a CT content did not affect (P> 0.05) rumen pH. The obtained rumen pH is approximately 6.86-6.92. This pH value is still in the optimal range for rumen microbial fermentation activity, where the normal rumen pH for rumen microbial activity is 6.0-7.0 (Grant and Martens, 1992). Meanwhile, for optimal rumen function, Kamra (2005) and Ososanya et al. (2013) state that rumen pH must range between 6.0-6.8.
The present study showed that increasing the supplementation of SL with a CT content linearly (P<0.05) decreased IVDMD and IVOMD. This result is in line with that obtained by Kondo et al. (2014) and Yuliani et al. (2014), where supplementation of tannins from lerak fruit (Sapindus rarak) and tea byproducts caused a decrease in IVDMD and IVOMD. Another study found that adding 2 mg/ml tannin during the 48 h incubation period significantly reduced IVDMD and IVOMD (P<0.05) (Yugianto et al., 2014). The decrease in IVDMD and IVOMD with increased supplementation of SL with a CT content is due to the role of tannins in forming complex bonds with carbohydrates and proteins. Stergiadis and Harvey (2017) state that tannins can bind to protein and polysaccharides so that the feed becomes difficult to degrade by rumen microbes and can cause a decrease in the value of feed digestibility. Bueno et al. (2020) found that tannins inhibited DMD and OMD through their interactions with proteins and polysaccharides. The reduction of DMD and OMD will positively impact an increase in protein and nonstructural carbohydrate flow to the small intestine. Min et al. (2006) state that formation complexes of CT with protein and polysaccharides thus increase the amount of protein with low rumen degradability that flows to the small intestine, favoring an increase in daily live weight gain and milk production.
The concentration of ammonia (N-NH3) in the rumen is influenced by the protein content and amino acids of the feed. Ammonia is formed from the process of deamination of amino acids by microbial activity so that the concentration is influenced by the digestible protein content in the feed. Most of the ammonia absorbed through the rumen wall will be used directly by rumen microbes to meet nitrogen needs (Yugianto et al., 2014). N-NH3 that was obtained in the present study ranged from 3.26 - 6.45 mM (5.54 - 10.97 mg/dl), and this value is still in the optimal range to meet the needs of N microbes in the rumen. According to Satter and Slyter (1974), a minimum N-NH3 concentration of 5 mg/dl is needed to support optimal rumen microbial growth. Increasing the supplementation of SL with a CT content linearly decreased (P<0.05) the ruminal N-NH3 concentration. The results of this study are in line with those reported by several researchers in which tannin administration significantly decreased N-NH3 concentrations (Sharifi et al., 2013; Bhatta et al., 2013; Yuliana et al., 2014; Jolazadeh et al., 2017). According to this study, CT from SL has the ability to protect dietary protein from ruminal degradation by forming tannin-protein complexes and could be used to increase bypass protein and to improve ruminant performance. When the sheep's diet was switched from perennial grass without the tannin content into Lotus corniculatus with CT (32 g of CT/kg of DM), the proteolytic bacteria Butyrivibrio fibrosolvens population decreased (Bhatta et al., 2009).
TVFA concentrations and TG values are presented in Table 2. TVFA and TG decreased (P < 0.05) with increasing the supplementation of SL with a CT content. TVFA obtained in this study varied from 123-178 mM. The TVFA value obtained is still optimal to meet the rumen microbial energy needs. Van Soest (1982) states that the concentration of TVFA needed to support optimal microbial growth ranges from 80 - 160 mM. Hungate (1966) stated that a TVFA concentration of 111 mM was the minimum value needed for rumen microbial growth. CT supplementation up to T3 treatment (6% CT) still did not disrupt the energy supply needed by rumen microbes. Bhatta et al. (2014) reported that tannins per se do not cause substantial inhibition of TVFA concentrations, which generally does not indicate specific inhibition of rumen fermentation activity. Although the inclusion of tannin plants affects the degradability of OM and NDF, the total and individual VFAs are relatively similar between feeds. This is consistent with the findings of several previous researchers (Hariadi and Santoso., 2010; Jayanegara et al., 2011; Bhatta et al., 2014; Pineiro-Vazquez et al., 2018).
Increasing the level of CT supplementation from SL effectively reduced GT production. The GT produced in this study varied from 51.1 to 74.45 ml/200 mg DM, where GT production was higher at T0 (P <0.05) than at T1, T2, and T3. Meanwhile, T1 treatment was higher (P <0.05) compared to T2, T3, and then between T2 and T3 were not different (P> 0.05) (Tabel 2, Figure 1). The decrease in gas production obtained in this study was due to decreased microbial enzyme activity, especially cellulase enzymes. Cellulase enzymes play a role in degrading the fraction of fibers that contribute to the production of fermented gas in the rumen. Bueno et al. (2020) found that tannins can decrease cumulative gas production by forming a tannin-macromolecule complex bond that inhibits microbial enzyme activities. Another study stated that a high tannin concentration in the diet may lead to a reduction in microbial enzyme activities, such as cellulase (Tabbaco et al., 2006).
The production of methane gas and the percentage of methane gas from total gas decreased significantly (P <0.05) with increasing levels of CT-SL supplementation. The total methane gas and methane gas percentages vary from 31.03 - 66.47 ml/200 mg DM and 60.63 - 89.29%, respectively. The methane reduction potential (MRP) of T3 (53.32%) and T2 (49.56%) was higher than that of T1 (27.82%). The methane production of T0 and T1 was higher (P <0.05) than that of T2 and T3, while that of T2 and T3 was not different (P> 0.05). Hariadi and Santoso (2010) also reported that CH4 production decreased with increasing concentrations of total tannins in plants. Cieslak et al. (2016) confirmed the same where increasing CT decreases TG and methane production. This study showed that CT from SL could act as an antimetanogenic compound by directly inhibiting methane production. On the other hand, CT was also decreasing in OMD and TVFA. This study corresponds to the findings of Jayanegara et al. (2009), Jayanegara and Palupi (2010), and Bueno et al. (2020) that tannins have a direct influence on methanogens, cellulolytic bacteria, and proteolytic bacteria as well as an indirect influence on decreasing OMD and TVFA production by inhibiting the hydrogen supply for methane production.
Conclusion: Sengon leaves (SL) with condensed tannin (CT) content could be used as a feed supplement to decrease methane production. Supplementation SL with CT content was more effective in decreasing total gas and methane production with a CT concentration of 4% (equal to 0.09 g SL/0.2 g BR or equal to supplementation of 45% SL from a dry feed basis) without a negative effect and was still favorable for IVDMD, IVOMD, N-NH3, and TVFA to ruminal microbial activities and ruminant animal needs. The results suggest that evaluation of in vivo conditions is needed.
Acknowledgments: This research was funded by the Ministry of Research, Technology and Higher Education, University of Jambi, through a senior lecturer research program with Contract Number: 274/UN21.17/PP. This research would not have been possible without the aid of the undergraduate students and the technical assistant of the laboratory of Faculty of Animal Science, University of Jambi.
REFERENCES
- Anas, M. A., L. M. Yusiati, A. Kurniawati, C. Hanim. (2015). Evaluation of Albazia chinensis as tannins source for in vitro methane production inhibitor agents sheep rumen liquor. The 6th International Seminar on Tropical Animal Production Integrated Approach in Developing Sustainable Tropical Animal Production. October 20-22, 2015, Yogyakarta, Indonesia.
- (2007). Official Methode of Analysis. 18th Ed. Association of Official Analytical Chemist. Gaithersburg, M.D. USA.
- Bhatta, R., Y. Uyeno, K. Tajima, A. Takenaka, Y. Yabumoto, I. Nonaka, O. Enishi, and M. Kurihara. (2009). Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 92:5512-5522.
- Bhatta, R., Saravanan, L. Baruah, K.T. Sampath and C.S. Prasad. (2013). Effect of plant secondary compounds on in vitro methane ammonia production and ruminal protozoa population. J. of Appl. Microb. 115: 455-465.
- Bhatta, R., Saravanan, L. Baruah and C.S. Prasad. (2014). Effects of graded levels of tannin-containing tropical tree leaves on in vitro rumen fermentation, total protozoa and methane production. J. of Appl. Microb. 118: 557-564.
- Bueno, I.C.S., R.A. Brandi, G.M. Fagundes, G. Benetel and J.P. Muir (2020). The Role of Condensed Tannins in the In Vitro Rumen Fermentation Kinetics in Ruminant Species: Feeding Type Involved. Animal. 10 (635): 1-11.
- Cieslak, A., P. Zmora, A. Matkowski, I. Nawrot-Hadzik, E. Pers-Kamczyc, M. El-Sherbiny1, M. Bryszak1 and M. Szumacher-Strabel. (2016). Tannins from Sanguisorba officinalis affect in vitro rumen Methane production and fermentation. The J. of Anim. & Plant Sci. 26(1): 54-62.
- Cottle, D. J., V. Nolan and S. G. Wiedemann. (2011). Ruminant enteric methane mitigation a review. Aust. J. Exp. Agric. 48: 21-27.
- General Laboratory Procedure. (1969). Department of Dairy Science, University of Wisconsin, Madison. USA.
- V., O.J. Babayemi and D. Demeyer, D. (2005). Estimation of direct and indirect gas production in syringe: a tool estimate short-chain fatty acid production the requires minimal laboratory facilities. Animal Feed Science and Technology. 5(1):197-210.
- Gemeda, B.S. and Hassen. (2015). Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical brose plants. Asian Australas. J. Anim. Sci.28 (2): 188-199.
- Grant, R.J., and D.R. Mertens. (1992). Influence of buffer pH and raw corn starch addition on in vitro fiber digestion kineticks. J. Dairy Sci. 75: 2762-2768.
- Hariadi, B.T. and Santoso, B. (2010). Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J. Sci. Food and Agric. 90 (3): 456-461.
- Hassanat, F and C. Benchaar. (2013). Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. of the of Food and Agric.93 (2): 332-339.
- Hristov, A.N. Oh, J. L. Firkins, J. Dijkstra, E. Kebreab, G. Waghorn, H. P. S. Makkar, A. T. Adesogan, W. Yang, C. Lee, P. J. Gerber, B. Henderson, and J. M. Tricarico. (2013). Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 91:5045–5069
- Hungate, R. E. (1966). The Ruminant and Its Microbes. Agricultural Experimental Station. University of California Academic Press. New York, San Fransisco, London.
- Jayanegara, A., N. Togtokhbayar. H.P.S., Makkar, and K. Becker. (2009). Tannins determined by various methods as predictors of methane production reduction potential of plants by an in vitro rumen fermentation system. Anim. Feed Sci. Technol. 150: 230–237.
- Jayanegara, A. and E. Palupi. (2010). Condensed tannin effects on nitrogen digestion in ruminant, A meta-analysis from in vitro and in vivo studies. Pet. 33: 176-181.
- Jayanegara, A., Wina, C.R. Soliva, S. Marquardt, M. Kreuzer, F. Leiber. (2011). Dependence of forage quality and methanogenic potential of tropical plants on their phenolic fractions as determined by principal component analysis. Animal Feed Science and Technology 163: 231–243.
- Jayanegara, A., F. Leiber, M. Kreuzer. (2012). Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants fromin vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 96: 365–375.
- Jayanegara, A., E. Wina, Takahashi. (2014). Meta-analysis on methane mitigating properties of saponin-rich sources in the rumen in vitro: influence of addition levels and plant sources. Asian-Aust. J. Anim. Sci. 27: 1426–1435.
- Jolazadeh, A., and T. Mohammadabadi. (2017). Effect of treated sunflower with tannin extracted from pistachio hulls on in vitro gas production and ruminal fermentation. J. Veterinary research Forum. 8 (3): 203-208.
- Kamra, D.N. (2005). Rumen microbial. special section: microbial diversity. Current Sci. 89: 124–135.
- Kennedy, P.M. and E. Charmley. (2012). Methane yields from Brahman cattle fed tropical grasses and legumes. J. Product. Sci. 52(4):225-239.
- Kondo, M., Y. Hirano, K. Kita, A. Jayanegara, and H.O. Yokota. (2014). Fermentation characteristics, tannin contents and in vitro ruminal degradation of green tea and black tea byproducts ensiled at different temperatures. Asian Australas. J. Anim. Sci. 27 (7): 937-945.
- Mayangsari, N.S., A. Subrata, M, Cristiyanto (2013). Effect tannin to protection of soy pulp protein on ammonia concentration, total protein production and percentage of rumen undegraded dietary protein in vitro. J. Anim. Agriculture. 2(1): 261-268.
- Min, B.R., E. Pinchak, R.C. Anderson. (2006). Effects of condensed tannin supplementation level on weight gain and in vitro and in vivo bloat precursors in steers grazing winter wheat. J. of Anim. Sci. 84 (9): 2546-2554.
- Min, B.R., S. Solaiman, E. Taha and J. Lee. (2015). Effect of plant tannin-containing diet on fatty acid Profile in meat goats. J. of Anim. Research and Nutr.; 1 (15): 1-7.
- Naumann, H.D., L.O. Tedeschi , W.E. Zeller, N.F. Huntley. (2017). The role of condensed tannins in ruminant animal production: advances, limitations and future directions R. Bras. Zootec., 46 (12) : 929-949.
- NATIONAL COUNCIL ON CLIMATE CHANGE (NCCC). 2010. Indonesia’s greenhouse gas abatement cost curve. p. 52.
- Ningrat, R.W.S., Zain, Erpomen and H. Suryani. (2017). Effects of Doses and Different Sources of Tannins on in vitro Ruminal Methane, Volatile Fatty Acids Production and on Bacteria and Protozoa Populations. Asian J. of Anim. Sci. 11: 47-53.
- Ørskov, E. R and P. McDonald. (1979). The estimation of protein degradability in the rumen from incubation measurement weighted according to rate passage. J. Agric. Sci., Cambridge 92: 499 – 503.
- Ososanya, T.O., O.T. Odubola, and A. Shuaib-Rahim. (2013). Intake, nutrient digestibility and rumen ecology of West African Dwarf sheep fed palm kernel oil and wheat offal supplemented diets. Int. J. Agric. Sci. 3: 380–386.
- Piñeiro-Vázquez, A.T., J.R. Canul-Solís, J.A. Alayón-Gamboa, A.J. Chay-Canul, Ayala-Burgos, C.F. Aguilar-Péreza, F.J. Solorio-Sánchez, J.C Ku-Vera. (2015). Potential of condensed tannins for the reduction of emissions of enteric methane and their effect on ruminant productivity. Article Rev. Arch. Med. Vet. 47: 263-272.
- Piñeiro-Vázquez, A.T., J.R. Canul-Solis, G.O. Jiménez-Ferrer, J.A. Alayón-Gamboa, A.J. Chay-Canul, A.J. Ayala-Burgos, C.F. Aguilar-Pérez, and J.C. Ku-Vera (2018). Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of rumen protozoa in heifers fed low-quality forage. Asian-Australas J. Anim. Sci. 31(11):1738-1746.
- Porter, L.J., L.N. Hrstich, B.G. Chan. (1986). The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin, Phytochemistry 25: 223-230.
- Puchala, R,. G. Animut, A.K. Patra, G.D. Detweiler, J.E. Wells, V.H. Varel, T. Sahlu. (2012). Methane emissions by goats consuming Sericea lespedeza at different feeding frequencies. Anim Feed Sci Technol 175: 76-84.
- Satter, L.D. dan L. L. Slyter. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. Brit. J. Nutr. 32:199-208.
- Sharifi, M., A.A. Naserian and H. Khorasani. (2013). Effect of Tannin Extract from Pistachio by Product on in vitro Gas Production. Iranian J. Appli. Anim. Sci. 3(4): 667-671.
- Subepang, S., T. Suzuki, T. Phonbumrung, and K. Sommart (2019). Enteric methane emissions, energy partitioning, and energetic efficiency of zebu beef cattle fed total mixed ration silage. Asian-Australas J. Anim. Sci. 32 ( 4): 548-555.
- Stergiadis, S. dan I. M. Harvey. (2017). Benefits of Plant Tannins on Ruminant Nutrition, Health & Environment. School of Agriculture, Policy and Development. University of Reading.
- Tabacco E., G. Borreani, G.M. Crovetto, G. Galassi, D. Colombo and L. Cavallarin. (2006). Effect of chestnut tannin on fermentation quality, proteolysis and protein rumen degradability of alfalfa silage. J. Dairy Sci. 89: 4736-4746.
- Tavendale, M.H., L.P. Meagher, D. Pacheco, N. Walker, G.T. Attwood and S. Sivakumaran. (2005). Methane production from in vitro rumen incubation with Lotus pendunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 123/124:403-419.
- Theodorou, M.K. and A.E. Brooks (1990). Evaluation of a new procedure for estimating the fermentation kinetics of tropical feeds. Contractor Report (EMC X0162) for the Natural Resources Institute, Chatham, UK.
- Tilley, J.M.A. and R.A. Terry. 1963. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 18:104-111.
- Ushlu, O.S., O. Kurt, E. Kaya, and A. Kamalak. (2018). Effect of species on chemical composition, metabolizable energy, organic matter digestibility and methane production of some legume plants grown in Turkey. J. of Appl. Anim. Research. 46 (1); 1158-1161.
- Van Soest, P. J. (1982). Nutritional Ecology of The R O & B Book. Co. Corvallis. United States of America.
- Vázquez,G., L.H. Medina, L.M. Benavides, A.L. Caratachea, G. S. Razo, A.J.A. Burgos, R.O. Rodriguez. (2016). Effect of fodder tree species with condensed tannin contents on in vitro methane production. Asian Australas. J. Anim. Sci. 29 (1): 73-79.
- Yugianto, A. Sudarman, E. Wina, and A. Jayanegara. (2014). Supplementation tannin and saponin extracts to diets with different forage to concentrate ratio on in vitro rumen fermentation and methanogenesis. J. Indonesian Trop. Anim. Agric. 39 (3): 144-151.
- Yuliana, P., E.B. Laconi, E. Wina and A. Jayanegara. (2014). Extraction of tannins and saponins from plant sources and their effects in in vitro methanogenesis and rumen fermentation. J. Indonesian Trop. Anim. Agric. 39 (2): 91-97.
|





