IMPACT OF CONSERVATION TILLAGE ON ORGANIC MATTER DYNAMICS IN LOESS DRYLAND SOIL, PUNJAB, PAKISTAN
I. Naz1, 2, S. S. Ijaz1, Mussie Y. Habteselassie2, M. Ansar3 and K. S. Khan1
1Institute of Soil Science, PMAS- Arid Agriculture University, Rawalpindi 46300, Pakistan
2Department of Crop and Soil Sciences, University of Georgia Griffin Campus, 1109 Experiment Street, Griffin, GA 30223, USA
3Department of Agronomy, PMAS-Arid Agriculture University, Rawalpindi 46300, Pakistan
Corresponding Author’s Email: iram.nazee@gmail.com
ABSTRACT
Conservation tillage and carbon sequestration are critical issues in rain-fed farming areas of Pakistan. Conservation tillage is not extensively used in developing countries on dryland soil where marginal farming is practiced. Therefore, primary purpose of this experiment was to determine the influence of conservation tillage practices on soil organic carbon (SOC), particulate organic matter (POC), mineral associated organic carbon (MOC), microbial biomass carbon (MBC) and dehydrogenase activity (Dha) in loess dry land Pothwar, Punjab, Pakistan. The tillage practices included zero tillage (ZT), minimum tillage (MT), reduced tillage (RT) and conventional tillage (CT) with mouldboardplough as a control in main plot and fallow-wheat (Triticumaestivum L.) and mungbean (Vigna radiate L.) crop rotation in sub plot as a split plot layout. The results indicated that ZT showed higher SOC (7.90 g kg-1), POC (2.35 g kg-1), MOC (5.1 g kg-1), MBC (359.37 μgg-1) and Dha (45.12 TPFμgg-1 dry weight) than CT. Among crop rotation, overall mungbean-wheat showed higher values as compared to fallow-wheat crop. The study indicated that conservation tillage practices with legume crop rotation have potential for improving soil organic carbon storage and hence carbon sequestration in soil.
Keywords: Conservation tillage; soil organic carbon; microbial biomass carbon; dehydrogenase enzyme; loess dryland; mungbean-wheat
https://doi.org/10.36899/JAPS.2022.5.0534
Published first online April 26, 2022
INTRODUCTION
Soil organic carbon (SOC) is important for climate regulation, as it stabilizes carbon and mitigates yield reduction (Lal, 2004a). Despite its importance, loss of soil SOC is occurring, causing decline in soil fertility (Yu et al., 2006). Soil organic carbon loss is exacerbated due to conventional tillage (CT) practices such as removal of crop residues after harvest and intensive use of mouldboard plough (MBP) that disturbs the soil (Zhang et al., 2015). Such intensive tillage practices also affect the rate of carbon sequestration in soil (Roldán et al., 2005) and increase emission of greenhouse gasses CO2(La Scala et al., 2008),N2O (Chatskikh and Olesen, 2007) and CH4(Li et al., 2011). Conventional tillage practices also cause degradation of soil structure (Willekens et al., 2014)and erosion that result in loss of SOC (Liu et al., 2010). Intensive tillage practices on large scale result in reduction of soil organic matter, soil quality, and fertility in arid and semi-arid conditions (Álvaro-Fuentes et al. 2013; Abdullah, 2014). Thus, depletion of SOC has become a threat to agricultural production. Therefore, to reverse the trend of SOC loss, it is crucial to prioritize the adoption of practices that increase SOC such as the “4 per 1000” Initiative (https:// www.4p1000.org/) aims. This initiative aims at promoting soil management practices e.g. adoption of cover crops and reduced tillage to effectively increase SOC (Lal, 2016). Proper management of land can increase SOC (Lal, 2014, Zhang and Ni, 2017) through organic matter input and improvement of soil structure by promoting aggregates stability (Deb et al., 2015).
Conservation tillage practice (minimum soil disturbance, direct drilling, zero/no-tillage etc) is considered to be effective in increasing SOC and decreasing erosion in Northeast China (Zhang et al., 2015). Conservation agriculture with proper crop residue management practices had been shown not only to increase SOC but also improve soil quality and reduced soil degradation (Awale et al., 2013). Many studies have reported an increase in SOC and carbon fractions under short and long term residue management, mulching, integrated nutrient management, and fertilization (Verma et al., 2013; Mi et al., 2016). In CT, intensive ploughing leaves less than 15 percent of the crop residue, whereas, in conservation tillage, 30% of crop residue is left on soil surface (CTIC, 2015). However, the effect of zero tillage (ZT) on SOC is not always clear and consistent. In some studies, no-tillage has increased SOC as compared to CT (West and Post, 2002; Ogle et al., 2005; Kumar et al., 2012) while in other studies that was not the case (Baker et al., 2007; Blanco-Canqui and Lal, 2008; Powlson et al., 2014). These differences might be due to variations in soil type, duration of tillage, cropping systems and environmental conditions (Blanco-Canqui, 2013). The effect of cropping systems on SOC is crucial as it affects the characteristics and amount of crop residue input (Yang and Kay, 2001; Zuber et al., 2015). Crop residues differ in chemical composition that mostly determines their rate of decomposition and incorporation to the soil organic matter pool (Ogle et al., 2012; Poeplau et al., 2015). Other useful practices, for instance, conversion of fallow land to agro forestry and horticultural or cropland, could improve SOC and fractions on long term basis due to higher input (aboveground and belowground biomass) of organic matter in soil (Ramesh et al., 2013; Ramesh et al., 2015).
SOC is composed of different compounds that vary from simple to complex molecules that differ in stability (Deb et al., 2015). SOC has three different pools/fractions: slow, passive and active (Paul and Clark 1996; Paul and Collins 1998).Soil microbial biomass carbon (MBC) and particulate organic carbon (POC) are included in the active pool that is labile and may turnover from days to years (Brady and Weil, 2008). The role of these fractions in nutrient turnover is essential. Changes caused by soil management practices are difficult to determine only through quantification of total SOC measurements (Haynes, 2005). Quantifying changes in labile carbon pool has shown help in detecting changes in soil quality (Wang et al., 2014; Awale et al., 2017). Thus, chemical, physical and biological fractions of SOC e.g. POC, MBC and mineral associated organic carbon (MOC) have received more attention as they are more sensitive to soil management practice than total SOC (Dou et al., 2008). MOC is that carbon fraction in soil organic matter pool, which is stabilized physically and chemically. It is considered a passive pool of carbon that takes long time for the turnover process (Marschner et al., 2008). The passive and slow pools show more resistance to decomposition and turnover from decades to centuries (Dumale et al., 2009). The bioavailability of these fractions for microbial decomposition is very low due to the long turnover time (Six et al., 2002; Benbi et al., 2014).
Enzymes are biological catalysts that speed up chemical reactions without being consumed by the reaction. These chemical reactions are essential for microbial processes in soil, organic waste decomposition, organic matter formation and soil structure stabilization (Dick et al., 1994).Soil enzymes are produced by plants, animals and microorganisms and may be present in dead cells and cell debris and are also adsorbed by clay or incorporated into humic substances (Allison, 2005). Soil enzymes and SOC pools especially labile pools are considered good indicators for short term impacts with soil management (Hok et al., 2018). Soil enzymes play a significant part in mineralization of organic matter (María et al., 2002). Soil enzyme activities are often used as indicators of microbial activity and nutrient cycling (Sinsabaugh et al., 2008).For instance, soil dehydrogenase activity (Dha) mediate soil organic matter oxidation via the transfer of electron and proton from substrates to acceptors. Soil Dha occurred intracellular in all living cells of microorganisms. Thus, it is a good indicator of microbial activity (Stȩpniewska and Wolińska, 2005)
Conservation tillage is practiced on 125 million hectares of land all over the world and makes up 9% of arable cropland worldwide (Kassam et al., 2012). North America has the most significant share at45%, followed by South America and Canada (32%), New Zealand and Australia (14%) and the rest of the world (9%) (Friedrich et al., 2012). In Asia, only 2.2 % of the agricultural lands are under conservation tillage (Derpsch and Friedrich, 2010). Little information exists regarding SOC pools and its sensitivity to change under different land management practices in South Asian countries such as Afghanistan and Pakistan. In Pakistan, most research on SOC is related to fertility and the amount of soil organic carbon. There is less work regarding agricultural management practices to increase the quantity and quality of SOC (Lal, 2004b).
The dryland agroecological system of Pakistan consists of Pothwar plateau (Rawalpindi, Jhelum and Attock districts), upland of Khyber Pakhtunkhwa, Balochistan plateau, and desert of Cholistan (Sharif et al., 2017). In these areas, dryland farming has some constraints including low rainfall, soil fertility, and weakly structured soil, degraded and low in SOC concentration. In dryland farming, farmers employ a more intensive cultivation method with MBP (traditionally, 8–10 ploughings) and commonly grow winter wheat crop and leave the land fallow for six months till the next winter crop to conserve soil moisture (Hassan et al., 2015).Sixty-five percent of farmers employ a wheat-maize (2-year system) rotation system where eighty percent of farmers practice summer fallow with wheat (Arif and Malik, 2009).
In dryland areas of Pakistan, most studies on conservation tillage are related to yield improvement, and less information is available in relation to soil organic carbon fractions (Niaz et al., 2017). Therefore, more information is needed on the relationship among conservation tillage practices, SOC and soil quality in these areas. In view of this scenario, our study was conducted to evaluate the impact of conservation tillage practices on SOC and its fractions and soil Dha activity in crop rotation (wheat and mungbean). It was hypothesized that organic C, carbon fractions and soil enzyme activity improve with conservation tillage practices.
MATERIALS AND METHODS
Description of Study Location: A two-year conservation tillage field study was established in 2016 at the research farm of Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Pakistan (latitude 33°36’0” N, longitude 73°02’0” E) in a sandy clay loam soil. The experimental site is located in semi-arid dryland Pothwar (elevated 517 m from sea level) in the northern Punjab area (Fig.1). The area of Pothwar is 28488.9 sq Km. The major crops in Pothwar include wheat, millet, gram, barley, groundnut, and maize. The fallow-wheat (FW) system and intensive MBP tillage are common practices in this area. In summer, the temperature is very hot (range from 36ºC to 42ºC) and can get as high as 48 ºC in the extreme case (Nizami et al., 2004). In winter, temperature ranges between 4 oC and 25 oC but can drop below freezing point (Hussain et al., 2003). Seventy percent of the annual precipitation (750-950 mm) occurs during the summer or monsoon season (June to August). These heavy rain events cause soil erosion (Shaheen et al., 2010). In this area, dryland farming (6% irrigated and 94% rain-fed) has been a common practice for centuries. The soil pH and ECwere7.89 and 0.60 dS m-1, respectively. The soil has 55.2% sand, 23.4% silt, 21.4% clay and 5.5 g/kg SOC.
Figure1. Location of study sites in thepothwar plateau, Punjab.
Experimental Design: Thirty-two experimental plots, each of size 29 m × 11 m, were prepared to accommodate four treatments with four replications. The basic layout of the experimental plots in the field was split-plot design under RCBD (randomized complete block design). The main plot treatments were tillage systems that include CT (conventional tillage) in which soil was ploughed with MBP followed by 8 cultivations with tine cultivator; Minimum Tillage (MT) in which field was ploughed with chisel plough at depth of 25 cm followed by 4 times cultivation with tine cultivator; Reduced Tillage (RT) in which the soil was ploughed with chisel plough one time at a depth of 45 cm and treated with roundup herbicide (Glyphosate @ 1 L acre-1) for weed control; and ZT in which the plots were undisturbed for entire fallow period and weeds were controlled with roundup. Crop residues were retained in ZT and RT plots after crop harvest, while crop residues were removed from the MT and CT treated plots. Subplot treatments were fallow-wheat (FW) and mungbean-wheat (MW). Wheat (Chakwal-97) crop was seeded in the mid of November and harvested in May. Summer crop (Mungbean MN-11) was planted at the end of June and harvested in September.
Soil Sampling: A composite soil sample was collected from each plot of the experimental site before planting in order to analyze the basic (chemical, biological and physical) properties of soil. Plastic bags were used to preserve the soil samples then immediately shipped to laboratory for analysis. The air-dried sub-sample was ground and sieved with 2.0 mm sieve to determine the soil organic carbon and fractions. For analysis of soil dehydrogenase enzyme activity, fresh soil samples were immediately stored in a plastic bag at 40Cand then analysed within ten days.
Soil Physicochemical Analysis: The fractions of carbon were determined as described in (Cambardella and Elliott 1992). Soil sample (25g) was suspended in sodium hexametaphosphate solution (200ml) and transferred into the flask (500ml) and shaked for 30 min. on a mechanical shaker. The coarse fraction was separated after washing of soil suspension via >53mm sieve. The soil samples that were sieved or passed through the sieve were mineral MOC, and those that remained on the sieve were POC. The carbon fractions were transferred into an oven to dry at 60 ºC for organic carbon analysis via wet oxidation method. SOC, POC, and MOC were determined using the wet oxidation method (Walkley, 1947). Briefly, 1gm of soil was added into 500ml conical flask with 10 ml of potassium dichromate (1N) and 20 ml H2SO4, mixed and left it for 30 minutes. Then, distilled water (200 ml) and 10 ml concentrated H3PO4were added and allowed to cool it. The indicator diphenylamine (10-12 drops) was added and titrated with (0.5M) ferrous ammonium sulfate solution till color changes from violet blue to green.
Microbial biomass carbon was determined withchloroform (CHCl3) fumigation extraction method. Briefly, one portion of soil (10g) was fumigated by placing in desicator with 30 ml alcohol free chloroform in another 50 ml beaker for 18-24 hours at 25 oC and samples were extracted with 50 ml 0.5 M K2SO4 for 30 min at 200 revolutions per minute and filtered. The other portion of 10g soil was also extracted in the same way but without the fumigation process and extract (4 ml) was mixed with 0.0667 M potassium dichromate (1 ml) andconcentrated (5 ml) Sulphuric acid H2SO4. An indicator O-phenanthroline monohydrate (3-4 drop) was used and samples were titrated with ferrous ammonium sulphate solution till color changes from green/ violet to red (Anderson and Ingram, 1993). Analysis of Soil Enzymes Activity Soil Dha was determined colorimetrically as described in Alef and Nannipieri (1995). Complete procedure was done in diffused light due to light sensitivity.
Statistical Analyses: The data collected for various characteristics was subjected to analysis of variance (ANOVA) using split plot design under RCBD with Statistics® 8.1 software and means were compared at 5% level of significance by least significance difference test (Steel et al., 1997).
RESULTS
Soil Organic Carbon: The tillage practices and crop rotations had significantly affected SOC.SOC was the highest in ZT followed by RT with crop residues retention and MT as compared to CT. The highest amount of SOC (p =0.0001) was observed in ZT (7.90 gkg-1) in MW and ZT (7.35 g kg-1) in FW crop residues, followed by RT (7.21 g kg-1) in MW and RT (6.86 g kg-1) in FW crop residues (Table 1). The pattern of SOC among tillage treatment was in this order ZT > RT > MT > CT. Among crop rotations, MW resulted in6.85 g kg-1soil SOC and FW in 6.57g kg-1.
Table 1. Soil organic carbon (g kg-1) changes in 0-15 cm soil with different conservation tillage and crop rotation practices: fallow-wheat (FW), mungbean-wheat (MW)
| |
|
2016-2017
|
2017-2018
|
|
Treatment
|
|
Summer
|
Winter
|
Summer
|
Winter
|
|
Conventional Tillage
|
MW
|
5.82 c
|
5.66 e
|
5.83 e
|
5.98 d
|
| |
FW
|
5.54 c
|
6.02 de
|
6.03 e
|
5.96 d
|
|
Minimum Tillage
|
MW
|
5.81 c
|
6.31 cd
|
6.59 cd
|
6.72 bc
|
| |
FW
|
5.72 c
|
5.89 de
|
6.24 de
|
6.31 cd
|
|
Reduced Tillage
|
MW
|
6.17 abc
|
6.50 bc
|
7.14 ab
|
7.21 b
|
| |
FW
|
6.03bc
|
6.10 cd
|
6.73 bcd
|
6.86 bc
|
|
Zero Tillage
|
MW
|
6.72 a
|
7.14 a
|
7.42 a
|
7.90 a
|
| |
FW
|
6.59 ab
|
6.72 ab
|
7.07 abc
|
7.35 ab
|
Means with different letters show significant differences (p< 0.05).
Mineral Associated Organic Carbon: By the end of first year, only tillage practices had affected the soil MOC (p = 0.0002) and crop rotation effects were non-significant. However, in the second year, tillage and crop rotation both significantly affected MOC. The highest amounts of MOC were observed in ZT (5.1 g kg-1) and RT (4.85 g kg-1) in MW. Similarly, ZT (4.85 g kg-1) and RT (4.57 g kg-1) resulted in higher values in FW crop residue retention and then in MT as compared to CT (p = 0.0003) (Figs.2 and 3). In second year, MW had higher soil MOC (4.59 g kg-1) than FW (4.36 g kg-1) rotation (p = 0.013).
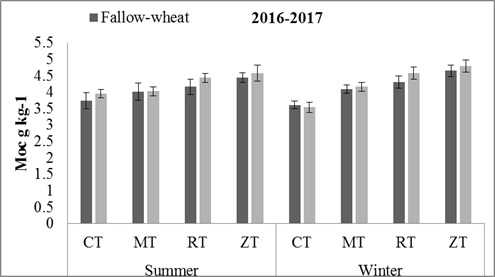
Figure 2. Soil MOC changes in 0-15 cm soil with different conservation tillage and crop rotation practices. Error bars in the mean values indicates the standard error. Abbreviations: MOC = mineral associated organic carbon,CT= conventional tillage, MT = minimum tillage, RT = reduced tillage, ZT = zero tillage.
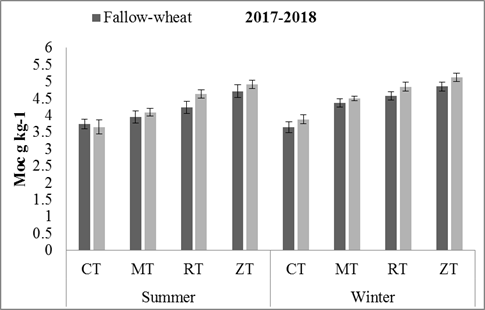
Figure 3. Soil MOC (mineral associated organic carbon) changes in 0-15 cm soil with different conservation tillage and crop rotation practices. Error bars in the mean values indicates the standard error. Abbreviations: MOC = mineral associated organic carbon, CT = conventional tillage, MT = minimum tillage, RT = reduced tillage, ZT = zero tillage.
Particulate Organic Carbon: ZT and RT with both crop residues retention significantly affected the soil POC as compared to other tillage treatments (p< 0.05) in both years. The highest POC (p= 0.0001) values were observed in ZT (2.35 gkg-1) and RT (2.29 g kg-1) at the end of the second year in MW residues retention. Similarly, soil POC was highest in ZT (2.21g kg-1) and RT (1.87 g kg-1) in FW residues retention, followed by MT as compared to CT. The lowest soil POC was determined in CT (1.02 g kg-1) treatment under FW rotation (Table 2). Among crop rotations, MW (1.77 g kg-1) rotation had significantly higher soil POC than FW (1.62 g kg-1) rotation (p = 0.012).
Table 2. Soil particulate organic carbon (g kg-1) changes in 0-15 cm soil with different conservation tillage and crop rotation practices: fallow-wheat (FW), mungbean-wheat (MW)
| |
|
2016-2017
|
2017-2018
|
|
Treatment
|
|
Summer
|
Winter
|
Summer
|
Winter
|
|
Conventional Tillage
|
MW
|
1.02 d
|
1.14 e
|
1.32 de
|
1.20 f
|
| |
FW
|
1.10 bcd
|
1.31 de
|
1.23 e
|
1.38 e
|
|
Minimum Tillage
|
MW
|
1.39 ab
|
1.44 cd
|
1.53 cd
|
1.66 cd
|
| |
FW
|
1.10 cd
|
1.24 e
|
1.32 e
|
1.56 de
|
|
Reduced Tillage
|
MW
|
1.53 a
|
1.72 ab
|
2.01 a
|
2.29 ab
|
| |
FW
|
1.35 abc
|
1.59 bc
|
1.60 bc
|
1.87 c
|
|
Zero Tillage
|
MW
|
1.65 a
|
1.91 a
|
1.86 ab
|
2.35 a
|
| |
FW
|
1.51 a
|
1.68 bc
|
1.81 ab
|
2.21 b
|
Means with different letters showed significant differences (P < 0.05).
Microbial Biomass Carbon: Soil MBC was also significantly affected by tillage and crop rotation in both years. Among different tillage systems, the highest values were observed in ZT (359.37 μgg-1) and (295.12 μg g-1) followed by RT (294.03 μg g-1) and (251.56 μg g-1) in MW and FW residues retention (p = 0.0003), respectively (Table 3). The lowest amount of MBC (141μg g-1) was observed in CT in the FW rotation. Among crop rotation, MW had shown (239.17 μg g-1) significantly higher (p = 0.009) value than FW (208.00 μg g-1).
Soil Dehydrogenase Enzyme Activity: The results showed that among tillage systems, soil Dha enzyme activity was significantly higher in ZT, RT and MT than control in first and second years. The highest enzyme activities (p = 0.001) were observed in ZT (45.12 TPF μg g-1(dwt) in MW and ZT (43.62 TPF μg g-1(dwt) in FW rotation (Table 4). The lowest activity was observed in CT (22.62 TPF μg g-1(dwt) in FW and CT (25.15 TPF μg g-1(dwt) in MW rotations. Among crop rotations, in the first year of experiment results were non-significant but in the second year, FW rotation had lower (p = 0.005) values (33.93 TPF μg g-1 (dwt) than MW (37.28 TPF μg g-1 (dwt).
Table 3. Soil Microbial biomass carbon (μgg-1) changes in 0-15 cm soil with different conservation tillage and crop rotation practices: fallow-wheat (FW), mungbean-wheat (MW)
| |
|
2016-2017
|
2017-2018
|
|
Treatment
|
|
Summer
|
Winter
|
Summer
|
Winter
|
|
Conventional Tillage
|
MW
|
153.55 bc
|
163.35 c
|
157.91 de
|
174.24 ef
|
| |
FW
|
138.30 c
|
143.75 c
|
141.57 e
|
147.02 f
|
|
Minimum Tillage
|
MW
|
163.35 bc
|
186.22 bc
|
186.22 cde
|
238.49 cd
|
| |
FW
|
152.46 bc
|
153.55 c
|
185.13 cde
|
206.91 de
|
|
Reduced Tillage
|
MW
|
185.13 ab
|
219.98 ab
|
229.78 abc
|
294.03 bc
|
| |
FW
|
175.33 bc
|
186.22 bc
|
196.02 bcd
|
251.56 bcd
|
|
Zero Tillage
|
MW
|
221.07 a
|
261.36 a
|
273.34 a
|
359.37 a
|
| |
FW
|
167.71 bc
|
217.80 b
|
240.67 ab
|
295.12 ab
|
Means with different letters showed significant differences (P < 0.05).
Table 4. Soil dehydrogenase activity (TPF μgg-1 (dwt)) changes in 0-15 cm soil with different conservation tillage and crop rotation practices: fallow-wheat (FW), mungbean-wheat (MW)
| |
|
2016-2017
|
2017-2018
|
|
Treatment
|
Crop rotaion
|
Summer
|
Winter
|
Summer
|
Winter
|
|
Conventional Tillage
|
MW
|
25.15 bc
|
27.12 e
|
29.54 cd
|
29.77 c
|
| |
FW
|
22.62 c
|
24.46 e
|
27.00 d
|
26.77 d
|
|
Minimum Tillage
|
MW
|
29.08 abc
|
29.08 cde
|
34.38 bc
|
37.84 b
|
| |
FW
|
27.12 abc
|
27.58 de
|
34.50 bc
|
33.12 c
|
|
Reduced Tillage
|
MW
|
31.62 ab
|
32.08 bcd
|
37.26 ab
|
42.69 a
|
| |
FW
|
28.62 abc
|
32.77 abc
|
29.54 cd
|
39.12 b
|
|
Zero Tillage
|
MW
|
32.77 a
|
37.62 a
|
41.65 a
|
45.12 a
|
| |
FW
|
33.92 a
|
34.62 ab
|
37.85 ab
|
43.62 a
|
Means with different letters showed significant differences (P < 0.05).
DISCUSSION
Present study recorded considerably higher SOC content in ZT with crop residues left after harvest, as compared to traditional intensive tillage (CT). The results are in agreement with findings of Sharif et al. (2015) who worked under similar semiarid conditions and found higher SOC, POC, MOC, MBC in ZT than the other tillage treatments. It has been repeatedly reported in semiarid areas that long term application of conservation tillage increased the SOC(Álvaro-Fuentes et al., 2008; Hernanz et al., 2009; Lopez-Fando and Pardo, 2011). The increase in SOC storage with ZT tillage is also supported by many other studies (Yeboah et al., 2016; Kumar et al., 2017; Zhang et al., 2018).
The slow rate of decomposition process in ZT resulted in accumulation of organic content within soil that cause an increase in SOC in the topsoil (Álvaro-Fuentes et al., 2008; Dikgwatlhe et al., 2014; Jat et al., 2019b).It is often reported that SOC concentration becomes higher due to interacting factors such as higher crop residue addition or retention, minimum soil disturbance, high moisture content, low risk of erosion and lower soil surface temperature(Logan et al., 1991; Ismail et al., 1994).The high SOC in reduced tillage system improved the sustainability of the system over the long term due to carbon sequestration. Crop residues are precursors for SOC pool. As such, they are linked with increase in SOC (Dolan et al., 2006). The effects of conservation tillage may vary with characteristics and the amount of crop residues returned to soil. Moreover, tillage causes redistribution of organic matter in the soil.
A small change in POC can be an early indication in improvement or degradation of soil regarding farm management practices(van Wesemael et al., 2019), especially in relation to soil disturbance(Chan et al., 2002), the quality, quantity and rate of decomposition of residues (Chivenge et al., 2007). POC exerted great effect on the ability of soil to supply nutrient and structural stability(Yoo and Wander, 2008).Thus, it is considered to play an important role in soil quality(Haynes, 2005). In this study, POC was also improved in ZT with residue retention. Results of this study are in line with the earlier studies that have recorded higher POC under no-tillage system with crop rotation as compared to the intensive tillage practices(Motta et al., 2007; Dou et al., 2008; Awale et al., 2013; Martin-Lammerding et al., 2013; Aziz et al., 2014; Wang and Sainju, 2014).These results are similar to the findings of other studies in same semiarid conditions (Virto et al., 2007; Álvaro-Fuentes et al., 2008). The high input of carbon within soil through crop residues left in field, recycling in ZT resulted in higher amount of POC (Yoo and Wander, 2008).
In this study, MOC was higher in ZT than conventional CT. Earlier studies also showed similar results in which MOC in reduced tillage management was higher than CT (Carpenter-Boggs et al., 2003; Mikha and Rice, 2004; Mahdi et al., 2005; Álvaro-Fuentes et al., 2008; Dou et al., 2008). In no-tillage practices, the higher MOC might be attributed to high carbon substrate availability for decomposition via microbial biomass (Chen et al., 2009).
Soil microbes turn over organic matter in soil (Mooshammer et al., 2014). In present study, ZT showed significantly higher MBC than CT. Similar results were obtained in other studies which reported significant increase in MBC under no-tillage with residue retention and crop rotation than CT (Bausenwein et al., 2008; Silva et al., 2010; Yeboah et al., 2016; Awale et al., 2017; Choudhary et al., 2018; Jat et al., 2019a). Govaerts et al. (2007b)performed a long-term experiment in rainfed conditions in Mexico to observe the tillage and residue management and crop rotation effect on soil MBC and microbial activity and found zero tillage resulted in either similar level or increased microbial biomass and activity as compared to conventional tillage.
The fact that no-tillage had higher MBC than CT might be due to high content of SOC and POC associated with no-tillage systems. Some studies reported that tillage practices had influenced MBC (Madejón et al., 2009; Melero et al., 2009). In no-tillage system, C immobilization increased by microbial biomass was attributed to an increase in organic matter in the form of plant residue(Bayer et al., 2002) while the reason for low MBC under CT was related to intensive soil disturbance and reduced plant coverage among other factors (Glover et al., 2000). No-tillage protects microbial habitat and decreases the extreme effect of temperature fluctuations (Rhoton, 2000). The soil cover and low soil disturbance protect microorganisms, in addition to improving nutrient availability for microbial activity and growth. Zero tillage, as a result of residue retention, improved water retention and infiltration (Govaerts et al., 2007a). Such benefit is crucial under water limiting conditions for agricultural systems in dryland rainfed areas.
In this study, SOC, MBC, POC and MOC were higher in wheat - mungbean rotation than fallow – wheat rotation. Soil biological properties improved with mungbean residues. MBC, microbial activity and mineralizable carbon were higher in mungbean residues receiving plots. Mungbean residues stimulated microbial activity and growth with mineralization of plant nutrients (Naeem et al., 2009). Tejada et al. (2009)also found that soil biological properties (enzymatic activity and MBC) were positively affected with plant residues. This might be due to more nitrogen fixation and nitrogen released from organic matter decomposition and subsequently incorporated into microbial biomass. The root nodulation and above ground residue after harvest of mungbean indicate the valuable source of nitrogen and organic matter. Their decomposition gives a meaningful contribution to nitrogen in soil. Such results were supported by many researchers (Tejada et al., 2006; Tejada and Gonzalez, 2006; Stark et al., 2007; Tejada et al., 2008).
The Dha was significantly affected by tillage management practices. Soil Dha activity was highest in conservation tillage (ZT) management practices than common traditional methods. These results are consistent with the results of Parihar et al., (2016) that performed a long term (field) experiment with different tillage practices and intensive use of crop rotation and reported higher Dha in ZT than CT in legume based crop rotation. The same findings were obtained by Madejón et al. (2007) and in rainfed conditions by Kumar et al.(2017).A review paper of 68 studies showed that residue retention and reduction in tillage intensity significantly improved soil Dha enzyme (Saikia and Sharma, 2017). The same was proven by the results of Wang et al.(2012) and Panettieri et al.(2013).The decomposition (residue) may release some nutrients (like nitrogen, phosphorus, and sulfur) necessary for plant and microbial growth (Jat et al., 2015). The higher SOC in conservation tillage system enhances soil carbohydrates that supply energy sources for soil microbes (Mina et al., 2008).The higher organic content caused an increase in soil dehydrogenase enzyme activity, resulting in decomposition of organic matter (Khan and Joergensen, 2009). The increased Dha in zero treatment might be due to more availability of labile carbon to the microbes compared with others (Jat et al., 2019a).Inclusion of pulse crops in the sequence can increase soil Dha in MW, followed by FW. This trend might be due to high SOC in legume-based crop sequence (Roa-Fuentes et al., 2015; Nawaz et al., 2017)
CONCLUSION
The conservation tillage and cropping system highly affected SOC fractions and soil dehydrogenase activity. It is clear from the experiment that zero tillage and reduced tillage, with residue retention, had higher SOC, POC, MOC and Dha than conventional tillage practices in loess dryland soil. So, less intensive tillage practice might be the practice to enhance the soil carbon storage that will ultimately influence soil quality and productivity.
Acknowledgments: We would like to thank Syed Amir Manzoor, University of Reading (UK) and Fawad Khan, University of Georgia, Griffin (U.S.A), for providing help in making the study area map and useful suggestion in editing the manuscript.
Disclosure statement: The authors declare no conflict of interest.
Funding: This work was financially supported by Higher Education Commission Pakistan (HEC) under grant number [3179].
REFERENCES
- Abdullah, A.S. (2014). Minimum tillage and residue management increase soil water content, soil organic matter and canola seed yield and seed oil content in the semiarid areas of Northern Iraq. Soil Tillage Res. 144:150–155.
- Alef, K. and P. Nannipieri (1995). Methods in applied soil microbiology and biochemistry. Academic Press; San Diego (USA). 229 p
- Allison, S.D. (2005). Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 8(6):626–635.
- Álvaro-Fuentes, J., M.V. López, C. Cantero-Martinez and J.L. Arrúe (2008). Tillage effects on soil organic carbon fractions in Mediterranean dryland agroecosystems. Soil Sci. Soc. Am. J. 72(2):541–547.
- Álvaro-Fuentes, J., F.J. Morell, E. Madejón, J. Lampurlanés, J.L. Arrúe and C. Cantero-Martínez (2013). Soil biochemical properties in a semiarid Mediterranean agroecosystem as affected by long-term tillage and N fertilization. Soil Tillage Res. 129:69–74.
- Anderson, J. M. and J. S. I. Ingram (1993). Tropical soil siology and fertility: Handbook of methods.CAB International; Wallingford Oxfordshire (UK) 68 p
- Arif, M. and M.A. Malik (2009). Economic feasibility of proposed cropping patterns under different soil moisture regimes of pothwar plateau. Int. J. Agric. Biol. 11(1):27–32.
- Awale, R., A. Chatterjee and D. Franzen (2013). Tillage and N-fertilizer influences on selected organic carbon fractions in a North Dakota silty clay soil. Soil Tillage Res. 134:213–222.
- Awale, R., M.A. Emeson and S. Machado (2017). Soil organic carbon pools as early indicators for soil organic matter stock changes under different tillage practices in Inland Pacific Northwest. Front. Ecol. Evol. 5:1–13.
- Aziz, I., T. Mahmood and K.R. Islam (2014). Impact of long-term tillage and crop rotation on concentration of soil particulate organic matter associated carbon and nitrogen. Pakistan J. Agric. Sci. 51(4):827–834.
- Baker, J.M., T.E. Ochsner, R.T. Venterea and T.J. Griffis (2007). Tillage and soil carbon sequestration-What do we really know? Agric. Ecosyst. Environ. 118(1–4):1–5.
- Bausenwein, U., A. Gattinger, U. Langer, A. Embacher, H.P. Hartmann, M. Sommer, J.C. Munch and M. Schloter (2008). Exploring soil microbial communities and soil organic matter: Variability and interactions in arable soils under minimum tillage practice. Appl. Soil Ecol. 40(1):67–77.
- Bayer, C., J. Mielniczuk, L. Martin-Neto and P.R. Ernani (2002). Stocks and humification degree of organic matter fractions as affected by no-tillage on a subtropical soil. Plant Soil. 238(1):133–140.
- Benbi, D.K., A.K. Boparai and K. Brar (2014). Decomposition of particulate organic matter is more sensitive to temperature than the mineral associated organic matter. Soil Biol. Biochem. 70:183–192.
- Blanco-Canqui, H. (2013). Crop residue removal for bioenergy reduces soil carbon pools: How can we offset carbon losses? Bioenergy Res. 6(1):358–371.
- Blanco-Canqui, H. and R. Lal (2008). No-tillage and soil-profile carbon sequestration: An on-farm assessment. Soil Sci. Soc. Am. J. 72(3):693–701.
- Brady, N.C., R.R. Weil (2008). The Nature and roperties of soils. 14th Ed. Prentice Hall; New Jersey (USA). 518 p
- Cambardella, C.A. and E.T. Elliott (1992). Particulate soil organic-matter changes across a grassland cultivation sequence. Soil. Sci. Soc. Am. J. 56:777–783.
- Carpenter-Boggs, L., P.D. Stahl, M.J. Lindstrom and T. E. Schumacher (2003). Soil microbial properties under permanent grass, conventional tillage and no-fill management in South Dakota. Soil Tillage Res. 71(1):15–23.
- Chan, K. Y., D.P. Heenan and A. Oates (2002). Soil carbon fractions and relationship to soil quality under different tillage and stubble management. Soil Tillage Res. 63(3–4):133–139.
- Chatskikh, D. and J.E. Olesen (2007). Soil tillage enhanced CO2 and N2O emissions from loamy sand soil under spring barley. Soil Tillage Res. 97(1):5–18.
- Chen, H., R. Hou, Y. Gong, H. Li, M. Fan and Y. Kuzyakov (2009). Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Tillage Res. 106(1):85–94.
- Chivenge, P.P., H.K. Murwira, K.E. Giller, P. Mapfumo and J. Six (2007). Long-term impact of reduced tillage and residue management on soil carbon stabilization: Implications for conservation agriculture on contrasting soils. Soil Tillage Res. 94(2):328–337.
- Choudhary, M., H.S. Jat, A. Datta, A.K. Yadav, T.B. Sapkota, S. Mondal, R.P. Meena, P.C. Sharma and M.L. Jat (2018). Sustainable intensification influences soil quality, biota, and productivity in cereal-based agroecosystems. Appl. Soil Ecol. 126:189–198.
- (2015). National crop residue management survey; Conservation technology information centre. http://www.ctic.purdue.edu.
- Deb, S., P.B. S. Bhadoria, B. Mandal, A. Rakshit and H.B. Singh (2015). Soil organic carbon: Towards better soil health, productivity and climate change mitigation. Clim. Chang. Environ. Sustain. 3(1):26–34.
- Derpsch, R. and T. Friedrich (2010). Sustainable crop production intensification.The adoption of conservation agriculture worldwide-. Proc. 16th ISCO Congr. Santiago (Chile).
- Dick, R. P., J.A. Sandor and N.S. Eash (1994). Soil enzyme activities after 1500 years of terrace agriculture in the Colca Valley, Peru. Agric. Ecosyst. Environ. 50(2):123–131.
- Dikgwatlhe, S.B., Z. Du Chen, R. Lal, H.L. Zhang and F. Chen (2014). Changes in soil organic carbon and nitrogen as affected by tillage and residue management under wheat-maize cropping system in the North China Plain. Soil Tillage Res. 144:110–118.
- Dolan, M.S., C.E. Clapp, R.R. Allmaras, J.M. Baker and J.A.E. Molina (2006). Soil organic carbon and nitrogen in a Minnesota soil as related to tillage, residue and nitrogen management. Soil Tillage Res. 89(2):221–231.
- Dou, F., A.L. Wright and F.M. Hons (2008). Sensitivity of labile soil organic carbon to tillage in wheat-based cropping systems. Soil Sci. Soc. Am. J. 72:1445–1453.
- Dumale, W.A., T. Miyazaki, T. Nishimura and K. Seki (2009). CO2 evolution and short-term carbon turnover in stable soil organic carbon from soils applied with fresh organic matter. Geophys. Res. Lett. 36(1):1–6.
- Friedrich, T., R. Derpsch and A. Kassam (2012). Overview of the global spread of conservation agriculture. Field. Actions Sci. Rep. 1–7.
- Glover, J.D., J.P. Reganold and P.K. Andrews (2000). Systematic method for rating soil quality of conventional, organic, and integrated apple orchards in Washington State. Agric. Ecosyst. Environ. 80(1–2):29–45.
- Govaerts, B., M. Fuentes, M. Mezzalama, J.M. Nicol, J. Deckers, J.D. Etchevers, B. Figueroa-Sandoval and K.D. Sayre (2007a). Infiltration, soil moisture, root rot and nematode populations after 12 years of different tillage, residue and crop rotation managements. Soil Tillage Res. 94(1):209–219.
- Govaerts, B., M. Mezzalama, Y. Unno, K.D. Sayre, M. Luna-Guido, K. Vanherck, L. Dendooven and J. Deckers (2007b). Influence of tillage, residue management, and crop rotation on soil microbial biomass and catabolic diversity. Appl. Soil Ecol. 37(1–2):18–30.
- Hassan, A., S.S. Ijaz, R. Lal, D. Barker, M. Ansar, S. Ali and S. Jiang (2015). Tillage effect on partial budget analysis of cropping intensification under dryland farming in Punjab, Pakistan. Arch. Agron. Soil Sci. 62:151–162.
- Haynes, R.J. (2005). Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 85:221–268.
- Hernanz, J.L., V. Sánchez-Girón and L. Navarrete (2009). Soil carbon sequestration and stratification in a cereal/leguminous crop rotation with three tillage systems in semiarid conditions. Agric. Ecosyst. Environ. 133(1–2):114–122.
- Hok, L., J.C. de Moraes Sá, M. Reyes, S. Boulakia, F. Tivet, V. Leng, R. Kong, C. Briedis, D. da Cruz Hartman, L.A. Ferreira, T.M. Inagaki, D.R.P. Gonçalves and P.T. Bressan (2018). Enzymes and C pools as indicators of C build up in short-term conservation agriculture in a savanna ecosystem in Cambodia. Soil Tillage Res. 177:125–133.
- Hussain, I., A.M. Cheema and A.A. Khan (2003). Small rodents in the crop ecosystem of Pothwar Plateau, Pakistan. Wildl. Res. 30:269.
- Ismail, I., R.L. Blevins and W.W. Frye (1994). Long-term no-tillage effects on soil properties and continuous corn yields. Soil Sci. Soc. Am. J. 58:193–198.
- Jat, H.S., A. Datta, M. Choudhary, P.C. Sharma, A.K. Yadav, V. Choudhary, M. K. Gathala, M.L. Jat and A. McDonald (2019a). Climate smart agriculture practices improve soil organic carbon pools, biological properties and crop productivity in cereal-based systems of North-West India. Catena. 181:1-12.
- Jat, H.S., A. Datta, M. Choudhary, A.K. Yadav, V. Choudhary, P.C. Sharma, M.K. Gathala, M.L. Jat and A. McDonald (2019b). Effects of tillage, crop establishment and diversification on soil organic carbon, aggregation, aggregate associated carbon and productivity in cereal systems of semi-arid Northwest India. Soil Tillage Res. 190:128–138.
- Jat, H.S., G. Singh, R. Singh, M. Choudhary, M.L. Jat, M.K. Gathala and D.K. Sharma (2015). Management influence on maize-wheat system performance, water productivity and soil biology.. Soil Use Manag. 31:534–543.
- Kassam, A., T. Friedrich, R. Derpsch, R. Lahmar, R. Mrabet, G. Basch, E. J. González-Sánchez and R. Serraj (2012). Conservation agriculture in the dry Mediterranean climate. Field. Crop. Res. 132:7–17.
- Khan, K.S. and R.G. Joergensen (2009). Changes in microbial biomass and P fractions in biogenic household waste compost amended with inorganic P fertilizers. Bioresour. Technol. 100(1):303–309.
- Kumar, A., A. Panda, L.K. Srivastava, and V.N. Mishra (2017). Effect of conservation tillage on biological activity in soil and crop productivity under rainfed Vertisols of central India. Int. J. Chem. Stud. 5(6):1939–1946.
- Kumar, S., A. Kadono, R. Lal and W. Dick (2012). Long-term no-till impacts on organic carbon and properties of two contrasting soils and corn yields in Ohio. Soil Sci. Soc. Am. J. 76(5):1798–1809.
- Lal, R. (2004a). Soil carbon sequestration impacts on global climate change and food security. Science. 304:1623–1627.
- Lal, R. (2004b). Carbon sequestration in soils of Central Asia. L. Degrad. Dev. 15(6):563–572.
- Lal, R. (2014). Soil carbon management and limate Change. In: Soil arbon. Springer International Publishing,Switzerland. p. 339–361.
- Lal, R. (2016). Beyond COP21: Potential and challenges of the “4 per Thousand” initiative. J. Soil Water Conserv. 71(1):20A-25A.
- La Scala, N., A. Lopes, K. Spokas, D. Bolonhezi, D.W. Archer and D.C. Reicosky (2008). Short-term temporal changes of soil carbon losses after tillage described by a first-order decay model. Soil Tillage Res. 99:108–118.Li, D., M. Liu, Y. Cheng, D. Wang, J. Qin, J. Jiao, H. Li and F. Hu (2011). Methane emissions from double-rice cropping system under conventional and no tillage in southeast China. Soil Tillage Res. 113:77–81.
- Liu, X.B., X.Y. Zhang, Y.X. Wang, Y.Y. Sui, S.L. Zhang, S.J. Herbert and G. Ding (2010). Soil degradation: a problem threatening the sustainable development of agriculture in Northeast China. Plant Soil Environ. 56:87 – 97.
- Logan, T. J., R. Lal and W.A. Dick (1991). Tillage systems and soil properties in North America. Soil Tillage Res. 20(2-4):241–270.
- Lopez-Fando, C. and M.T. Pardo (2011). Soil carbon storage and stratification under different tillage systems in a semi-arid region. Soil Tillage Res. 111(2):224–230.
- Madejón, E., F. Moreno, J.M. Murillo and F. Pelegrín (2007). Soil biochemical response to long-term conservation tillage under semi-arid Mediterranean conditions. Soil Tillage Res. 94(2):346–352.
- Madejón, E., J.M. Murillo, F. Moreno, M.V. López, J.L. Arrue, J. Alvaro-Fuentes and C. Cantero (2009). Effect of long-term conservation tillage on soil biochemical properties in Mediterranean Spanish areas. Soil Tillage Res. 105(1):55–62.
- Mahdi, M., Al-Kaisi and X. Yin (2005). Tillage and crop residue effects on soil carbon and carbon dioxide emission in corn-soybean rotations. J. Environ. Qual. 34(2):437–445.
- María, D. L.P. J., A.M. De la Horra, L. Pruzzo and R.M. Palma (2002). Soil quality: A new index based on microbiological and biochemical parameters. Biol. Fertil. Soils. 35:302–306.
- Marschner, B., S. Brodowski, A. Dreves, G. Gleixner, A. Gude, P.M. Grootes, U. Hamer, A. Heim, G. Jandl, R. Ji, K. Kaiser, K. Kalbitz, C. Kramer, P. Leinweber, J. Rethemeyer, A. Schäffer, M.W.I. Schmidt, L. Schwark and G.L.B. Wiesenberg (2008). How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 171(1):91–110.
- Martin-Lammerding, D., J.L. Tenorio, M. Albarran, E. Zambrana and I. Walter (2013). Influence of tillage practices on soil biologically active organic matter content over a growing season under semiarid Mediterranean climate. Spanish J. Agric. Res. 11(1):232–243.
- Melero, S., K. Vanderlinden, J.C. Ruiz and E. Madejn (2009). Soil biochemical response after 23 years of direct drilling under a dryland agriculture system in southwest Spain. J. Agric. Sci. 147(1):9–15.
- Mi, W., L. Wu, P.C. Brookes, Y. Liu, X. Zhang and X. Yang (2016). Changes in soil organic carbon fractions under integrated management systems in a low-productivity paddy soil given different organic amendments and chemical fertilizers. Soil Tillage Res. 163:64–70.
- Mikha, M.M. and C. W. Rice (2004). Tillage and manure effects on soil and aggregate-associated carbon and nitrogen, Soil Sci. Soc. Am. J. 68:809–816.
- Mina, B. L., S. Saha, N. Kumar, A.K. Srivastva and H.S. Gupta (2008). Changes in soil nutrient content and enzymatic activity under conventional and zero-tillage practices in an Indian sandy clay loam soil. Nutr. Cycl. Agroecosystems. 82:273–281.
- Mooshammer, M., W. Wanek, I. Hämmerle, L. Fuchslueger, F. Hofhansl, A. Knoltsch, J. Schnecker, M. Takriti, M. Watzka, B. Wild, K.M. Keiblinger, S. Zechmeister-Boltenstern and A. Richter (2014). Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 5:1–7.
- Motta, A.C.V., D.W. Reeves, C. Burmester and Y. Feng (2007). Conservation tillage, rotations, and cover crop affecting soil quality in the tennessee valley: Particulate organic matter, organic matter, and microbial biomass. Commun. Soil Sci. Plant Anal. 38(19–20):2831–2847.
- Naeem, M., F. Khan and W. Ahmad (2009). Effect of farmyard manure , mineral fertilizers and mung bean residues on some microbiological properties of eroded soil in district Swat. Soil Env., 28(2):162–168.
- Nawaz, A., M. Farooq, R. Lal, A. Rehman, T. Hussain and A. Nadeem (2017). Influence of Sesbania brown manuring and Rice residue mulch on soil health, weeds and system productivity of conservation rice–wheat systems. L. Degrad. Dev. 28:1078–1090.
- Niaz, S., S.S. Ijaz, A. Hassan and M. Sharif (2017). Landuse impacts on soil organic carbon fractions in different rainfall areas of a subtropical dryland. Arch. Agron. Soil Sci. 63:1337–1345.
- Nizami, M. I., M. Shafiq, M. Aslam and A. Rashid (2004). The Soils and their agricultural development potential in Pothwar. NARC, Islamabad.
- Ogle, S.M., F.J. Breidt and K. Paustian (2005). Agricultural management impacts on soil organic carbon storage under moist and dry climatic conditions of temperate and tropical regions . Biogeochemistry. 72(1):87–121.
- Ogle, S. M., A. Swan and K. Paustian (2012). No-till management impacts on crop productivity, carbon input and soil carbon sequestration. Agric. Ecosyst. Environ. 149:37–49.
- Panettieri, M., H. Knicker, A.E. Berns, J.M. Murillo and E. Madejón (2013). Moldboard ploughing effects on soil aggregation and soil organic matter quality assessed by 13C CPMAS NMR and biochemical analyses. Agric. Ecosyst. Environ. 177:48–57.
- Parihar, C.M., M.R. Yadav, S.L. Jat, A.K. Singh, B. Kumar, S. Pradhan, D. Chakraborty, M.L. Jat, R.K. Jat, Y.S. Saharawat and O.P. Yadav (2016). Long term effect of conservation agriculture in maize rotations on total organic carbon, physical and biological properties of a sandy loam soil in north-western Indo-Gangetic Plains. Soil Tillage Res. 161:116–128.
- Paul, E.A. and H.P. Collins (1998). The characteristics of soil organic matter relative to nutrient cycling: Methods for assessment of soil degradation. CRC Press; Boca Ratón. 181–198 p
- Paul, E.A. and F.E. Clark (1996). Soil microbiology and biochemistry.2nd Ed. Academic Press; San Diego. 71-106 p
- Poeplau, C., H. Aronsson, Å. Myrbeck and T. Kätterer (2015). Effect of perennial ryegrass cover crop on soil organic carbon stocks in southern Sweden. Geoderma Reg. 4:126–133.
- Powlson, D.S., C.M. Stirling, M.L. Jat, B.G. Gerard, C.A. Palm, P.A. Sanchez and K.G. Cassman (2014). Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 4(8):678–683.
- Ramesh, T., K. M. Manjaiah, K.P. Mohopatra, K. Rajasekar and S.V. Ngachan (2015). Assessment of soil organic carbon stocks and fractions under different agroforestry systems in subtropical hill agroecosystems of north-east India. Agrofor. Syst. 89(4):677–690.
- Ramesh, T., K.M. Manjaiah, J.M.S. Tomar and S.V.Ngachan (2013). Effect of multipurpose tree species on soil fertility and CO2 efflux under hilly ecosystems of Northeast India. Agrofor. Syst. 87(6):1377–1388.
- Rhoton, F.E. (2000). Influence of time on soil response to no-till practices. Soil Sci. Soc. Am. J. 64:700–709.
- Roa-Fuentes, L.L., C. Martínez-Garza, J. Etchevers and J. Campo (2015). Recovery of soil C and N in a Tropical pasture: Passive and active restoration. L. Degrad. Dev. 26:201–210.
- Roldán, A., J.R. Salinas-García, M.M. Alguacil and F. Caravaca (2005). Changes in soil enzyme activity, fertility, aggregation and C sequestration mediated by conservation tillage practices and water regime in a maize field. Appl. Soil Ecol. 30(1):11–20.
- Saikia, R. and S. Sharma (2017). Soil enzyme activity as affected by tillage and residue management practices under diverse cropping systems. Int. J. Curr. Microbiol. Appl. Sci. 6(10):1211–1218.
- Shaheen, A., M.A. Naeem, G. Jilani and M. Shafiq (2010). Integrated soil management in eroded land augments the crop yield and water-use efficiency. Soil Plant Sci. 60(3):274–282.
- Sharif, M., S. Sohail Ijaz, S. Ali, M. Ansar and A. Hassan (2015). Buildup of soil organic carbon and stable aggregates under conservation tillage in loess dryland soil. J. Biol. Environ. Sci. 6(1):446–453.
- Sharif, M., S. Sohail Ijaz, M. Ansar, R. Latif, A. Hassan and M. Nasir (2017). Conservation griculture: esearch status, opportunities and challenges in dryland areas of pakistan. J. Biol. Environ. Sci. 11(3):102–112.
- Silva, A. P., L.C. Babujia, J. C. Franchini, R.A. Souza and M. Hungria (2010). Microbial biomass under various soil- and crop-management systems in short- and long-term experiments in Brazil. Field. Crop. Res. 119(1):20–26.
- Sinsabaugh, R. L., C.L. Lauber, M.N. Weintraub, B. Ahmed, S.D. Allison, C. Crenshaw, A.R. Contosta, D. Cusack, S. Frey, M.E. Gallo, T.B. Gartner, S.E. Hobbie, K. Holland, B.L. Keeler, J.S. Powers, M. Stursova, C. Takacs-Vesbach, M.P. Waldrop, M.D. Wallenstein, D.R. Zak and L.H. Zeglin (2008). Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 11(11):1252–1264.
- Six, J., R.T. Conant, E.A. Paul and K. Paustian (2002). Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil. 241(2):155–176.
- Stark, C., L M. Condron, A. Stewart, H. J. Di, and M. O’Callaghan (2007). Influence of organic and mineral amendments on microbial soil properties and processes. Appl. Soil Ecol. 35(1):79–93.
- Stȩpniewska, Z. and A. Wolińska (2005). Soil dehydrogenase activity in the presence of chromium (III) and (VI). Int. Agrophysics. 19(1):79–83.
- Steel, R. G., J.H. Torrie and D.A. Dickey (1997). Principles and procedures of statistics: A biological approach. Third Ed. McGraw Hill Book Company; New York. 334-381 p.
- Tejada, M., C. Garcia, J.L. Gonzalez and M.T. Hernandez (2006). Organic amendment based on fresh and composted beet vinasse: Influence on soil properties and wheat yield. Soil Sci. Soc. Am. J. 70(3):900–908.
- Tejada, M., and J.L. Gonzalez (2006). Crushed cotton gin compost on soil biological properties and rice yield. Eur. J. Agron. 25(1):22–29.
- Tejada, M., J.L. Gonzalez, A.M. García-Martínez and J. Parrado (2008). Application of a green manure and green manure composted with beet vinasse on soil restoration: Effects on soil properties. Bioresour. Technol. 99(11):4949–4957.
- Tejada, M., M.T. Hernandez and C. Garcia (2009). Soil restoration using composted plant residues: Effects on soil properties. Soil Tillage Res. 102(1):109–117.
- Verma, B.C., S.P. Datta, R.K. Rattan and A.K. Singh (2013). Labile and stabilised fractions of soil organic carbon in some intensively cultivated alluvial soils. J. Environ. Biol. 34(6):1069–1075.
- Virto, I., M. J. Imaz, A. Enrique, W. Hoogmoed and P. Bescansa (2007). Burning crop residues under no-till in semi-arid land, Northern Spain—effects on soil organic matter, aggregation, and earthworm populations. Soil Res. 45(6):414-421.
- Walkley, A. (1947). A critical examination of a rapid method for determining organic carbon in soils—effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 63(4):251–264.
- Wang, J. J., X.Y. Li, A.N. Zhu, X.K. Zhang, H.W. Zhang and W.J. Liang (2012). Effects of tillage and residue management on soil microbial communities in north china. Plant, Soil Environ. 58(1):28–33.
- Wang, J., and U.M. Sainju (2014). Soil carbon and nitrogen fractions and crop yields affected by residue placement and crop types. PLoS One. 9(8):e105039.
- Wang, Quanying, Y. Wang, Qicun Wang and J. Liu (2014). Impacts of 9 years of a new conservational agricultural management on soil organic carbon fractions. Soil Tillage Res. 143:1–6.
- van Wesemael, B., C. Chartin, M. Wiesmeier, M. von Lützow, E. Hobley, M. Carnol, I. Krüger, M. Campion, C. Roisin, S. Hennart and I. Kögel-Knabner (2019). An indicator for organic matter dynamics in temperate agricultural soils. Agric. Ecosyst. Environ. 274:62–75.
- West, T. O. and W.M. Post (2002). Soil organic carbon sequestration rates by tillage and crop rotation: A global data analysis a review. Soil Sci. Soc. Am. J. 66(6):1930–1946.
- Willekens, K., B. Vandecasteele, D. Buchan and S.De Neve (2014). Soil quality is positively affected by reduced tillage and compost in an intensive vegetable cropping system. Appl. Soil Ecol. 82:61–71.
- Yang, X.M. and B.D. Kay (2001). Rotation and tillage effects on soil organic carbon sequestration in a typic Hapludalf in southern Ontario. Soil Tillage Res. 59(3–4):107–114.
- Yeboah, S., R. Zhang, L. Cai, L. Li, J. Xie, Z. Luo, J. Liu and J. Wu (2016). Tillage effect on soil organic carbon, microbial biomass carbon and crop yield in spring wheat-field pea rotation. Plant, Soil Environ. 62(6):279–285.
- Yoo, G. and M. M. Wander (2008). Tillage effects on aggregate turnover and sequestration of particulate and humified soil organic carbon. Soil Sci. Soc. Am. J. 72(3):670–676.
- Yu, G., H. Fang, L. Gao and W. Zhang (2006). Soil organic carbon budget and fertility variation of black soils in Northeast China. Ecol. Res. 21(6):855–867.
- Zhang, G.S. and Z. W. Ni (2017). Winter tillage impacts on soil organic carbon, aggregation and CO2 emission in a rainfed vegetable cropping system of the mid–Yunnan plateau, China. Soil Tillage Res. 165:294–301.
- Zhang, S., X. Chen, S. Jia, A. Liang, X. Zhang, X. Yang, S. Wei, B. Sun, D. Huang and G. Zhou (2015). The potential mechanism of long-term conservation tillage effects on maize yield in the black soil of Northeast China. Soil Tillage Res. 154:84–90.
- Zhang, Y., X. Li, E. G. Gregorich, N. B. McLaughlin, X. Zhang, Y. Guo, A. Liang, R. Fan and B. Sun (2018). No-tillage with continuous maize cropping enhances soil aggregation and organic carbon storage in Northeast China. Geoderma. 330:204–211.
- Zuber, S. M., G.D. Behnke, E.D. Nafziger and M.B. Villamil (2015). Crop rotation and tillage effects on soil physical and chemical properties in Illinois. Agron. J. 107(3):971–978.
|





